How the NHS-Galleri trial has been designed
Key takeaway
The NHS-Galleri trial is looking at how useful a blood test is for finding cancer early in the NHS in England. The trial invited people from many different backgrounds and ethnicities, and put things in place so it would be simple for all types of people to take part.
Why finding cancer early is important
Around 140,000 people died from cancer each in England in 2019.[1] Finding cancer early often means it can be treated more successfully. The NHS wants to find 75% of cancers at an early stage by 2028.[2] However only around half of cancers were found at an early stage in England in 2017.[3]
How cancer is currently found
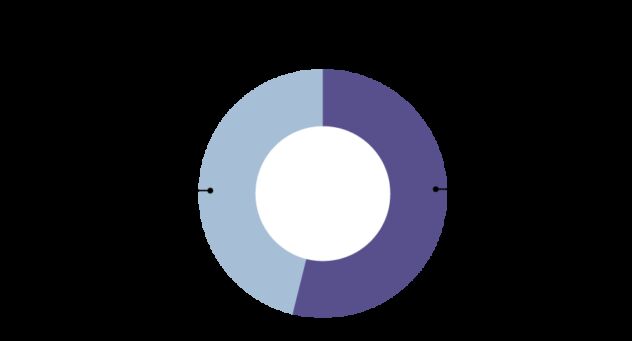
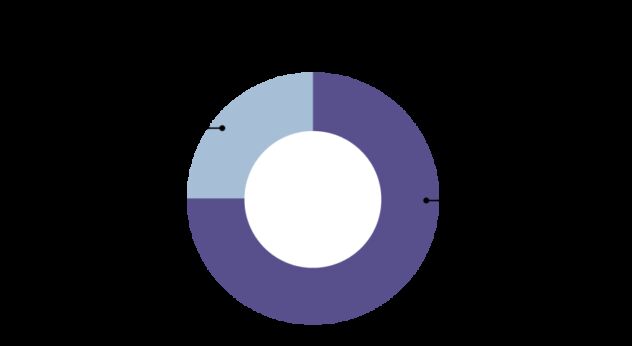
Cancer screening can help to find cancers early. The NHS in England has three national screening programmes, for breast, bowel and cervical cancer.[4] However the majority of people who die from cancer in the UK have a cancer for which no screening test is available.[5]
 The NHS in England has three national screening programmes.
The NHS in England has three national screening programmes.
Screening for multiple cancers
The Galleri® test is a new blood test that can detect a signal shared by many different types of cancer in a sample of a person’s blood.
A ‘Cancer Signal Detected’ result is not a diagnosis of cancer. Further tests are needed to confirm if a person has cancer.
The NHS-Galleri trial
The NHS-Galleri trial is a large research trial taking place across eight regions in England. The trial aims to find out if screening with the Galleri test can help the NHS to find cancer early, when used alongside existing cancer screening.
The NHS-Galleri trial is a randomised controlled trial. Randomised controlled trials compare two groups of people to understand how well a new test or treatment works. One of these groups does get the new test or treatment, and the other does not. These are called the ‘test’ and ‘control’ groups.
- What outcome will be assessed? If people in the test group have less cancers diagnosed at a late stage than the control group.
- What will be measured? Number of people who have cancer diagnosed and at what stage.
- Why are we interested in assessing cancers diagnosed at a late stage? For most cancer types, the stage at diagnosis is strongly linked with patient survival.[6]
Why is the trial taking place in England?
England has a national healthcare system — the NHS — where everyone receives the same standard of care. This enables:
- Recruitment of a large number of people
- Simple follow-up of people taking part, both during and after the trial
- The trial to be carried out quickly.
How people were invited to take part
Around 1.5 million people were invited to take part in the NHS-Galleri trial.
People could take part in the trial if they were:
- Aged 50–77
- Registered with a General Practitioner (GP) in one of eight regions in England
- Did not have cancer, or were not undergoing tests to see if they might have cancer
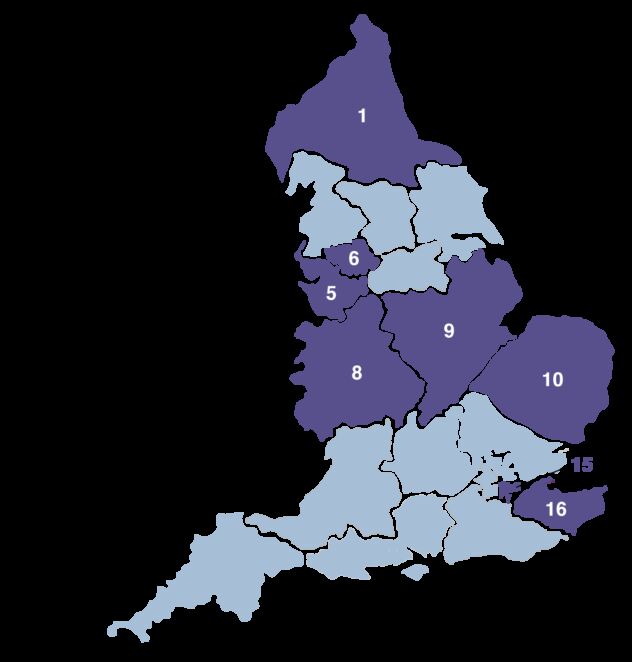 The NHS-Galleri trial is taking place in eight regions in England.
The NHS-Galleri trial is taking place in eight regions in England.
The trial invited people from many different backgrounds and ethnicities, including people with health conditions (not cancer) who are usually left out of clinical trials. This was to make sure the study represents all people aged 50–77 living in the UK.
The researchers regularly checked the age, gender and ethnicity of people who had signed up for the trial, as well as several measures of how well off their area was. The balance of people sent invitations was changed if it looked like low numbers of people from certain groups had signed up. This was to make sure there was good representation across each group.
What people taking part were asked to do
People who wanted to take part in the NHS-Galleri trial were asked to attend an appointment at a mobile clinic visiting their area. The mobile clinics were based at locations that should be easy for people to travel to by car or public transport, such as supermarket car parks. Other things, like offering interpreters and providing wheelchair and step-free access, helped many people take part in the trial.
Once someone agreed to take part, a blood sample was taken and some health surveys completed. People were then put at random into the test group or the control group. There was a 50:50 chance (like flipping a coin) of being in either group.
People in the test group will:
- Have their blood samples tested using the Galleri test
- Be told if a cancer signal is detected
- Not be told any other results.
People in the control group will:
- Not have their blood samples tested — they will be stored and may be tested in the future
- Not be told any results.
A control group is needed in research like this to provide a comparison with the test group.
In the NHS-Galleri trial, half of the people in the trial are in the test group and half in the control group. People in the trial do not know whether they are in the test or control group. This is because if people know which group they are in, it could change the way they behave about their health. This could make the research results less clear or reliable.
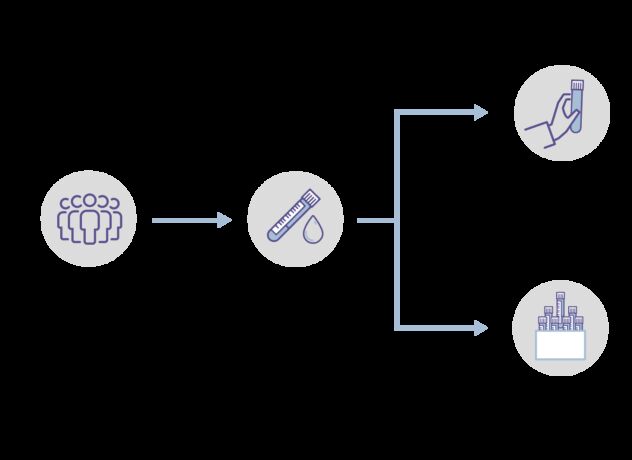
People in both groups are reminded that it is important to attend their usual cancer screening appointments they have been invited to, and to report any new or unusual symptoms to their GP.
Blood is collected from people up to three times in the trial:
- At their first appointment, when they join the trial
- At their second (12 month) appointment
- At their third (24 month) appointment.
This mirrors yearly (or annual) screening.
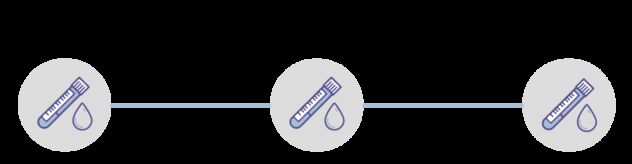 Blood samples are collected at three appointments over two years, about 12 months apart.
Blood samples are collected at three appointments over two years, about 12 months apart.
Health information, such as whether someone developed cancer and how it was treated, is collected from centrally held NHS records for up to 10 years after people’s first appointment. This allows the researchers to easily track people for whether they get cancer, even for people who may have moved home.
People who are diagnosed with cancer while they are taking part in the trial may not need to attend further trial appointments to give blood samples.
What happens if a cancer signal is found in someone’s blood sample?
In the test group, a small number of tests are expected to show a cancer signal.
A ‘Cancer Signal Detected’ result does not mean someone has cancer. It just means that they might have cancer and further tests are needed to check.
People who have a ‘Cancer Signal Detected’ will be told about their result by a trial nurse, who will arrange an appointment at an NHS hospital for further tests.
Further tests could include scans, scopes or more blood tests. NHS England has made guidance to support hospitals arranging further tests after a cancer signal has been detected.
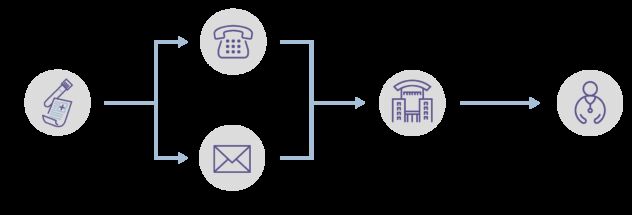 People in the test group who have a cancer signal detected will receive a phone call from a trial nurse to tell them a signal has been found. A letter will also be sent to them and their GP. An appointment will be arranged at an NHS hospital for further tests to check for cancer.
People in the test group who have a cancer signal detected will receive a phone call from a trial nurse to tell them a signal has been found. A letter will also be sent to them and their GP. An appointment will be arranged at an NHS hospital for further tests to check for cancer.
Most people on the trial will not get a test result. Only people in the test group who have a ‘Cancer Signal Detected’ will be given their test result. People in the test group whose blood sample shows no cancer signal will not be given a test result.
Summary of “Cell-Free DNA–Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial”
References
- Office for National Statistics. Mortality Statistics — Underlying Cause, Sex and Age. https://www.nomisweb.co.uk/query/construct/summary.asp?mode=construct&version=0&dataset=161#(accessed on 25 November 2022). Back
- NHS England. National Health Service Long Term Plan 2019. https://www.longtermplan.nhs.uk/(accessed on 25 November 2022). Back
- National Cancer Registration and Analysis Service. Stage Breakdown by CCG 2017. http://www.ncin.org.uk/publications/survival_by_stage(accessed on 25 November 2022). Back
- Cancer Research UK. What Is Cancer Screening? https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/spot-cancer-early/screening/what-is-cancer-screening(accessed on 25 November 2022). Back
- Cancer Research UK Cancer Mortality for Common Cancers. https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality/common-cancers-compared#heading-Zero(accessed on 25 November 2022). Back
- McPhail, S et al. Stage at Diagnosis and Early Mortality from Cancer in England. Br. J. Cancer 2015;112:S108–S115. DOI: 10.1038/bjc.2015.49. Back
